Methods for chemical analysis of rare earth impurities in rare earth metals and their oxides
Application scheme for determination of lanthanum, cerium, praseodymium, neodymium, Samarium, europium, Gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium content in yttrium (ICP-AES)
Methods According to GB/T 18115.12-2006
This method is suitable for the determination of lanthanum oxide, cerium oxide, praseodymium oxide, neodymium oxide, samarium oxide, europium oxide, gadolinium oxide, terbium oxide, dysprosium oxide, holmium oxide, erbium oxide, thulium oxide, ytterbium oxide and lutetium oxide in yttrium oxide. The measurement range is shown in Table 1.
The method is also applicable to lanthanum and cerium in yttrium. Determination of contents of neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium.
Form 1
|
Oxide |
Mass fraction/% |
Oxide |
Mass fraction/% |
|
lanthanum oxide |
0.0002~0.050 |
terbium oxide |
0.0003~0.050 |
|
Plutonium oxide |
0.0003~0.050 |
dysprosium oxide |
0.0002~0.050 |
|
praseodymium oxide |
0.0003~0.050 |
holmium oxide |
0.0003~0.050 |
|
neodymium oxide |
0.0003~0.050 |
Oxidized bait |
0.0002~0.050 |
|
samarium oxide |
0.0003~0.050 |
thulium oxide |
0.0002~0.050 |
|
europium oxide |
0.0002~0.050 |
ytterbium oxide |
0.0002~0.050 |
|
gadolinium oxide |
0.0002~0.050 |
lutecia |
0.0002~0.050 |
Method and principle
The samples were dissolved in hydrochloric acid, excited directly by argon plasma light source in dilute hydrochloric acid medium, and the influence of matrix on the determination was corrected by matrix matching method.
Instruments, reagents and materials
Macylab ICP-6810 inductively coupled plasma spectrometer, resolution <0.006 nm(200 nm). Argon plasma light source.
Hydrogen peroxide (30%). Hydrochloric acid (1+1). Hydrochloric acid (1+19).
Nitric acid (1+1). Argon ( > 99.99%).
Yttrium oxide matrix solution: Weigh 250,000 gyttrium oxide ( >99.999%) burned at 900C for 1 h, place it in 250 mL beaker, add 70 mL hydrochloric acid, heat it at low temperature until completely dissolved, cool it to room temperature, transfer it to 250 mL volumetric bottle, dilute it with water to the scale, and mix well. This solution contains 100 mg of yttrium oxide in 1mL.
Standard lanthanum oxide storage solution: Weigh 0.1000g lanthanum oxide ( >99.99%) burned at 900C for 1 h, place it in 100 ml beaker, add 10 mL hydrochloric acid (1+1), heat it at low temperature until completely dissolved, cool it to room temperature,transfer it to 100 mL volumetric bottle, dilute it with water to the scale, and mix well. This solution contains 1 mg lanthanum oxide in 1 mL. This solution was then diluted with hydrochloric acid (1+19) into 1 mL of 100ug and 1 mL of 10ug lanthanum oxide standard solutions.
Cerium oxide standard storage solution: Weigh 0.1000g cerium oxide ( >99.99%) burned at 900C for 1 h and place it in 100 ml. Add 10 mL nitric acid into the beaker, heat at low temperature, add hydrogen peroxide until completely dissolved, cool to room temperature, transfer to a 100 mL volumetric bottle, dilute with water to the scale, and mix well. This solution contains 1 mg cerium oxide in 1 mL. This solution is then diluted with hydrochloric acid into 1 mL containing 100 ug and 1 mL containing 10 ug cerium oxide standard solutions.
Standard storage solution of praseodymium oxide: Weigh 0.1000 g of praseodymium oxide ( >99.99%) burned at 900C for 1 h, place in 100 mL beaker, add 10 ml hydrochloric acid (1+1), heat at low temperature until completely dissolved, cool to room temperature, transfer to 100 mL volumetric bottle, dilute with water to scale, and mix well. The solution l mL contained 1 mg praseodymium oxide. Then this solution was diluted with hydrochloric acid (1+19) into 1 mL containing 100 ug and 1 mL containing 10ug praseodymium oxide standard solutions.
Neodymium oxide standard storage solution: Weigh 0.1000 g neodymium oxide burned at 900C for 1 h ( >99.99%). Place in a 100 mL glass, add 10 mL hydrochloric acid (3.2), heat at low temperature until completely dissolved, cool to room temperature, and transfer to 100 ml. In volumetric bottle, dilute to scale with water and mix well. This solution contains 1 mg neodymium oxide in 1 ml. This solution was then diluted with hydrochloric acid (1-19) into 1 ml of standard solution containing 100 μg and 1 mL of neodymium oxide containing 10 ug.
Samarium oxide standard storage solution: Weigh 0.100 0 g of samarium oxide ( >99.99%) burned at 900C for 1 h, place it in a 100mL beaker, add 10 ml hydrochloric acid (1+1), heat it at low temperature until it is completely dissolved, cool it to room temperature, transfer it to a 100 ml volumetric bottle, dilute it with water to the scale, and mix well. This solution contains 1 mg samarium oxide in 1 ml. This solution is then diluted with hydrochloric acid (1-19) into 1 mL. Standard solution containing 100 ug and 1 mL containing 10 ug samarium oxide.
Europium oxide standard storage solution: Weigh 0.100 0 g europium oxide ( >99.99%) burned at 900C for 1 h and place it in 100 mL. In the beaker, add i0 mL hydrochloric acid (1+1), heat at low temperature until completely dissolved, and cool to room temperature. Transfer to 100 mL volumetric bottle, dilute with water to scale, mix well. This solution 1 mL contains 1 mg europium oxide. This solution was then diluted with hydrochloric acid (1+19) into 1 mL of 100ug and 1 mL of 10 μg europium oxide standard solutions.
Gadolinium oxide standard storage solution: Weigh 0.100 0 g of gadolinium oxide ( >99.99%) that has been burned with 900 heart for 1 h, place it in a 100 mL bethel, add 10 ml hydrochloric acid (1+1), heat it at low temperature until completely dissolved, cool it to room temperature, transfer it to a 100 mL volumetric bottle, dilute it with water to the scale, and mix well. This solution contains 1 mg gadolinium oxide in 1 mL. This solution is then diluted with hydrochloric acid (1+19) to a standard solution of 1 mI. 100 ug and 1 mL 10 ug gadolinium oxide.
Terbium oxide standard storage solution: Weigh 0.100 0 g terbium oxide ( >99.99%) that has been burned at 900C for 1 h, place it in a 100 mL bethel, add 10 mL nitric acid (1+1), heat it at low temperature until it is completely dissolved, cool it to room temperature, transfer it to a 100 mL volumetric bottle, and dilute it to the scale with water. Mix well. This solution contains 1 mg terbium oxide in 1 mL. This solution is then diluted with hydrochloric acid (1+19) into 1 mL of 100 ug terbium and 1 mL of 10 ug terbium oxide standard solutions.
Dysprosium oxide standard storage solution: Weigh 0.1000g dysprosium oxide ( >99.99%) burned at 900℃ for 1 h, place in 100 ml beaker, add 10 mL hydrochloric acid (1+1), heat at low temperature until completely dissolved, cool to room temperature, transfer to 100 mL volumetric bottle, dilute with water to scale, and mix. Even. This solution contains 1 mg dysprosium oxide in 1 mL. This solution was then diluted with hydrochloric acid (1+19) into 1 mL containing 100 pg and 1 mL containing 10 ug dysprosium oxide standard solutions.
Holmium oxide standard storage solution: Weigh 0.100 g holmium oxide ( >99.99%) burned at 900℃ for 1 h and place it in 100 mL beaker. Add 10 mL hydrochloric acid (1+1), heat at low temperature until completely dissolved, and cool to room temperature. Transfer to 100 mL volumetric bottle, dilute with water to scale, mix well. This solution contains 1 mg holmium oxide in 1 ml. This solution was then diluted with hydrochloric acid (1+19) into 1 mL of 100 μg and 1 mL of 10 ug holmium oxide standard solutions.
Standard storage solution of erbium oxide: Weigh 0.0100 g of erbium oxide ( >99.99%) burned at 900℃ for 1 h, place it in 100 mL beaker, add 10 mL hydrochloric acid (1+1), heat it at low temperature until it is completely dissolved, and cool it to room temperature. Transfer to 100 mL volumetric bottle, dilute with water to scale, mix well. This solution contains 1 mg of erbium oxide in 1 mL. This solution was then diluted with hydrochloric acid (1+19) into 1 mL containing 100 pg and 1 mL containing 10 ug erbium oxide standard solutions.
Standard storage solution of thulium oxide: Weigh 0.100 0 g of thulium oxide ( > 99.99%) that has been burned at 900℃ for 1 h, place it in 100 mL beaker, and add 10 mI. Hydrochloric acid (1+1), heat at low temperature until completely dissolved, cool to room temperature, transfer to 100 mL volumetric bottle, dilute with water to scale, mix well. This solution contains 1mg of thulium oxide in 1mL. This solution is then diluted with hydrochloric acid (1+19) into 1mL of 100ug and 1 ml of 10ug of thulium oxide standard solutions.
Standard storage solution of ytterbium oxide: Weigh 0.100 0 g ytterbium oxide ( >99.99%) that has been burned at 900℃ for 1 h, place it in a 100 mL beaker, add 10 mL hydrochloric acid (3.2), heat it at low temperature until completely dissolved, cool it to room temperature, transfer it to a 100 mL volumetric bottle, dilute it with water to the scale, and mix well. The solution I mL contained 1 mg ytterbium oxide. This solution was then diluted with hydrochloric acid (3.3) into 1 mL of 100 ug and 1 mL of 10 ug of ytterbium oxide standard solutions.
Standard lutetium oxide storage solution: Weigh 0.100 0 g of lutetium oxide ( >99.99%), burned at 900℃ for 1 h, and place it in 100 ml. Add 10 mL hydrochloric acid (1+1) to the beaker, heat at low temperature until completely dissolved, cool to room temperature, transfer to a 100 mL volumetric bottle, dilute with water to the scale, and mix well. This solution contains 1 mg lutetium oxide in 1 mL. This solution is then diluted with hydrochloric acid (1+19) to 1 mL of 100 ug and 1 mL of 10ug lutetium oxide standard solutions.
Analysis procedure
Sample (1) The oxide sample was burned at 900 ° C for 1h, placed in a dryer, cooled to room temperature, and weighed immediately.
Sample (2) The surface oxide layer of the metal sample should be removed and weighed immediately after sampling.
specimen
The oxide sample is weighed with 0.500 g sample (1), accurate to 0.000 1 g.
Metal sample
Weigh the 0.394 g sample (2), accurate to 0.0001 g.
Number of measurements
Two samples were weighed, measured in parallel, and their average values were taken.
Analyze the preparation of test solution
Place the sample (6.1) in a 100 mL beaker, add 10 mL water, add 10 mL hydrochloric acid (3.2), heat at low temperature until completely dissolved, cool to room temperature, dilute with water in a 50 mL volumetric bottle to the scale, mix well, and set aside.
Preparation of standard series solutions
The yttrium oxide matrix solution and each rare earth oxide standard solution were transferred into 5 100 ml volumetric bottles and added 10 mI according to Table 2. Hydrochloric acid (1+1), diluted with water to the scale, mixed, prepared a standard series of solutions, ready for use.
Form 2 (continue)
|
Standard solution Number |
Mass concentration of each rare earth (as oxide) /ug/mL |
||||||
|
terbium oxide |
dysprosium oxide |
holmium oxide |
Oxidized bait |
thulium oxide |
ytterbium oxide |
Lutecia |
|
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
3 |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
|
4 |
5.00 |
5.00 |
5.00 |
5.00 |
5.00 |
5.00 |
5.00 |
|
5 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
Form 2
|
Standard solution Number |
Mass concentration of each rare earth (as oxide) /ug/mL |
|||||||
|
yttrium oxide |
lanthanum oxide |
cerium oxide |
praseodymium oxide |
neodymium oxide |
samarium oxide |
europium oxide |
gadolinium oxide |
|
|
1 |
10000 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
10000 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
3 |
10000 |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
2.00 |
|
4 |
10000 |
5.00 |
5.00 |
5.00 |
5.00 |
5.00 |
5.00 |
5.00 |
|
5 |
10000 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
The recommended analysis lines are shown in Table 3.
Form 3
|
Element |
analytical line/nm |
Element |
analytical line/nm |
|
La |
408.671 |
Tb |
350.917 |
|
Ce |
418.660 |
Dy |
353.170 |
|
Pr |
422.533 |
Ho |
345.600,339.898 |
|
Nd |
401.225 |
Er |
337.271 |
|
Sm |
428.078 |
Tm |
313.126 |
|
Eu |
381.965 |
Yb |
328.937 |
|
Gd |
342.246 |
Lu |
261.542 |
Argon plasma spectrometry was performed on the analytical solution and the standard series solution simultaneously.
The presentation of the analysis results
The content of the standard series solution is directly input into the computer, and according to the strength values of the standard series solution and the analytical test solution, the mass concentration of the rare earth element cable to be measured in the analytical test solution (6.3) is calculated, corrected and output by the computer.
The mass fraction (%) of rare earth elements to be tested is calculated as follows:
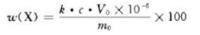
In the formula
k- The conversion coefficient of each element element and its oxide is shown in Table 4. When calculating the oxide content,k =1; c-- The mass concentration of rare earth oxides measured from the working curve, in micrograms per milliliter ( μg/mL);
V0 - total volume of test liquid, in milliliters (mL); m0-- The mass of the sample, in grams (g).
Form 4
|
Element |
k |
Element |
k |
|
La |
0.5826 |
Tb |
0.8502 |
|
Ce |
0.8140 |
Dy |
0.8713 |
|
Pr |
0.8277 |
Ho |
0.8730 |
|
Nd |
0.8573 |
Er |
0.8745 |
|
Sm |
0.8624 |
Tm |
0.8756 |
|
Eu |
0.8636 |
Yb |
0.8782 |
|
Gd |
0.8676 |
Lu |
0.7894 |
Instrument parameter
ICP-6810 Full spectrum direct reading inductively coupled plasma emission spectrometer

1. Product overview
ICP-6810 is a full-spectrum direct reading inductively coupled plasma emission spectrometer used to determine the content of trace and trace elements in different substances (soluble in nitric acid, hydrochloric acid, hydrofluoric acid, etc.), widely used in environmental protection, petroleum products, rare earth, semiconductor, geology, metallurgy, chemical industry, clinical medicine, food, biological samples, criminal science, agricultural research and other fields.
About us
Shanghai Macylab Instrument company profile
Shanghai Macylab Instrument Co., LTD. (hereinafter referred to as Macylab), is a high-tech enterprise with independent intellectual property rights, Macylab's entrepreneurial philosophy "science and technology - because you change", and take this as the enterprise purpose, continuous exploration, bold innovation. Especially in the field of analytical testing instruments, we have continuously developed advanced products, making us a supplier of high-quality instrument resources.

Macylab Main spectral instruments visible spectrophotometer, UV-visible spectrophotometer, atomic absorption spectrometer, ultramicrospectrophotometer, atomic fluorescence photometer, ICP inductively coupled plasma emission spectrometer, ICP inductively coupled plasma mass spectrometer, at present, Our products have been widely used in organic chemistry, inorganic chemistry, biochemistry, medicine, environmental protection, metallurgy, petroleum, agriculture and other fields. At the same time, with the rich experience accumulated in product mechanical structure, optical design, electrical application and software development, combined with the latest practical needs of the market, the company will launch a number of new analytical instruments in the near future.

Macylab's headquarters and production base are located in Shanghai, the marketing center is located in Beijing, and the company has R & D bases in Jiangsu, Shanghai and Shandong. In order to make full use of the intellectual resources around the country, the United States has also carried out in-depth scientific research cooperation with some scientific research institutions at home and abroad, and constantly transform scientific research achievements into productive forces. In order to better serve our customers, Meisei has 12 offices in China to customize application solutions that meet your needs and improve the added value of products. In the continuous service of domestic users at the same time, Macylab has also established a deep strategic cooperation relationship with more than 20 countries.
(Macylab Instrument Inc. is not only a high-tech certification enterprise, but also passed the CE certification, FCC certification, RoHS certification and a number of domestic qualification examination certification, and has a number of self-developed spectrum patents and Copyrights, etc.)



